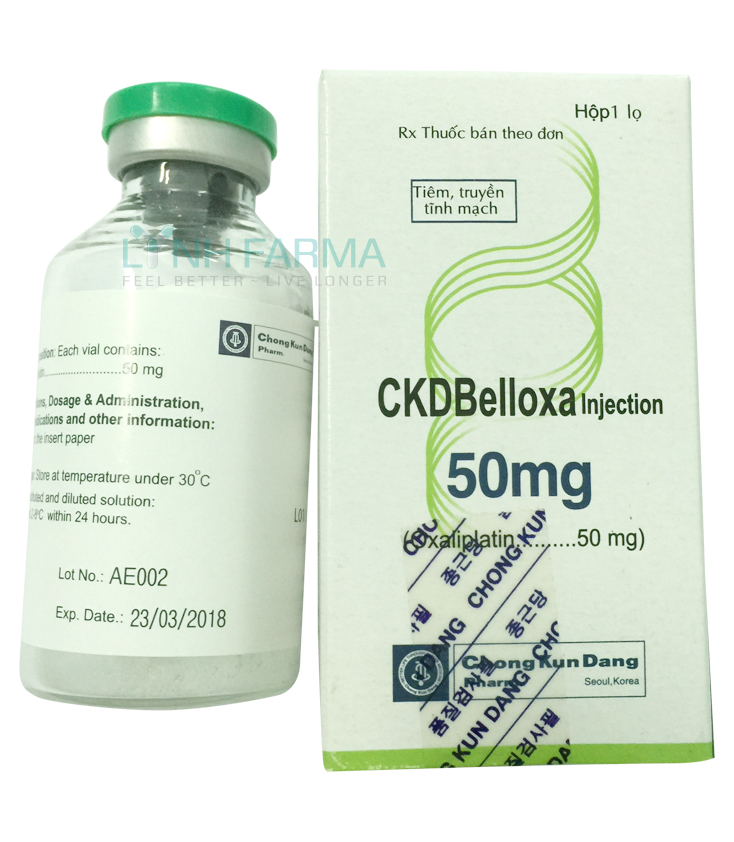
CKD Belloxa injection 50mg
Lyophilized powder for injection white.
Oxaliplatin in combination with 5-fluorouracil and folinic acid is indicated for use in:
- Adjuvant treatment of colon cancer stage III (Dukes stage C) after complete resection of primary tumor.
- Treatment of colorectal cancer metastases.
Price :
YOU NEED TO HELP ?
HOTLINE: 0917 55 70 88
- Company address: 49A30 Phan Dang Luu,Ward 7, Phu Nhuan District, Ho Chi Minh City, Vietnam.
- Email: info@lynhfarma.com
HOTLINE: 0917 55 70 88
- Company address: 49A30 Phan Dang Luu,Ward 7, Phu Nhuan District, Ho Chi Minh City, Vietnam.
- Email: info@lynhfarma.com
- Product Information
- Ingredient
CKD BELLOXA INJECTION, 50mg
Each vial contains:
- Oxaliplatin ......................50 mg
- Excipients: Lactose......... 450 mg
DESCRIPTION DOSAGE: lyophilized powder for injection white.
POINT:
Oxaliplatin in combination with 5-fluorouracil and folinic acid is indicated for use in:
- Adjuvant treatment of colon cancer stage III (Dukes stage C) after complete resection of primary tumor.
- Treatment of colorectal cancer metastases.
DOSAGE AND USE:
The recommended dose of oxaliplatin to 5-FU cooperate with or without the presence of folinic acid 85 mg / m2 body intravenously every 2 weeks. The treatment protocol is as follows:
Day 1: Transfer of 85 mg/m2 of oxaliplatin and 200mg/m2 leucovorin in 02 hours at the same time but different Y-line package and use, then transmits 5- fluorouracil (5-FU) concentration of 400 mg/m2 then adjust concentration of 600 mg/m2 for 22 hours.
Day 2: Transfer of 200 mg/m2 leucovorin for 02 hours then passed 5- fluorouracil (5-FU) concentration of 400 mg/m2 then adjust the concentration of 600mg/m2 for 22 hours. Recommended antiemetic pre-blockers and 5-HT3 receptors may be taken with or without dexamethasone.
How to prepare a solution:
Reconstituted solution:
Lyophilized powder for injection is diluted with distilled water for injection or 5% glucose solution to form a solution containing 5 mg oxaliplatin / ml.
- Oxaliplatin 50 mg: 10 ml diluted with solvent.
To ensure safety in terms of microbiological and chemical, after the solution should be used to revert immediately. Visual inspection prior to use, only when a clear solution without residue.
Intravenous infusion solution:
Dilute reconstituted with 5% glucose solution 250-500ml, to a solution with a concentration between 0.2mg/ml - 0.7mg/ml, peripheral intravenous central or in 2-6 hours, and always transferred 5-fluorouracil before transmission.
NOT AGAIN OR PHASE WITH DRUG DILUTION sodium chloride solution or solutions containing chloride.
The drug can only be used once. The remaining amount of the drug as well as all materials used for dilution or the infusion must be destroyed according to hospital procedures.
DOSE ADJUSTMENT:
The symptoms of peripheral neuropathy is caused by the toxicity of the dose, the dose of oxaliplatin adjustments will then be used depending on the degree of stretching and severity of these symptoms: If symptoms last lasted over 7 day and annoying, oxaliplatin dose reduction to 25% if paresthesia without causing dysfunction lasted until the next cycle, oxaliplatin dose reduction to 25%. If paresthesia accompanied dysfunction exists to the next cycle of treatment should be discontinued. If the symptoms improve after the drug is discontinued, it should consider taking back. When using oxaliplatin coordinate fluorouracil, dose 5-fluorouracil common toxicity should be adjusted. In addition of oxaliplatin dose should be reduced by 25% if there is diarrhea, decreased neutrophils, thrombocytopenia level 3-4.
CONTRAINDICATIONS:
Contraindications for the following cases: Patients with a history of severe allergic reaction to the drug Patients with peripheral nerve disease severe renal dysfunction: creatinine clearance Pregnant women and lactating
THE WARNINGS AND PRECAUTIONS: Use CKDBELLOXA injections under the control of a doctor with experience in chemotherapy. The nervous reactions are dose limited toxicity when treated with oxaliplatin. Oxaliplatin is related to two types of peripheral neuropathy (see undesirable effects). So be cautious while taking oxaliplatin. Ice (used in cases of talks) should be avoided during transmission oxaliplatin, because cold temperatures can exacerbate acute symptoms of neuropathy. Oxaliplatin is considered to be related to pulmonary fibrosis (<1% of patients), which may be fatal. Overall incidence of cases of cough, dyspnea and hypoxemia is 43% (for all authoritarian) and 7% (3 and 4 toxicity) in the oxaliplatin group combines 5-FU / LV compared with 32% (all toxicity) va5% (toxicity 3 and 4) in the irinotecan group combines 5-FU / LV, the patients did not determine the duration of illness and untreated cancer-direct colon before. In the case of the respiratory symptoms of unknown etiology such as cough without phlegm, shortness of breath, wheezing, or signs of abnormal chest radiographs, oxaliplatin is not recommended until the review more screening test to exclude pulmonary interstitial lung disease or pulmonary fibrosis. Aluminium is considered to cause degradation or disable platinum compound, so do not use needles or intravenous infusion sets containing aluminum parts can be exposed to the drug to be mixed or injected. Should not be used without dilution oxaliplatin. Oxaliplatin should not be used in conjunction with other drugs or infusion should not simultaneously with other medications in the same line. Precautions oxaliplatin for patients with a history of allergic reaction to platinum compounds. Incompatibility with the drug Oxaliplatin alkali or alkaline solution, so it should not be used with sodium chloride oxaliplatin or any fluids containing chloride. Check the solution of the strange particles and discoloration before use. Observe carefully before using the drug vial, the vial is used only during free of debris.
INTERACTIONS WITH OTHER MEDICINES:
Oxaliplatin cholinergic syndrome due to the presence irinotecan irinotecan acetylcholinesterase inhibitors. Oxaliplatin did not affect the pharmacokinetics of fluorouracil and topotecan. Preclinical studies have demonstrated oxaliplatin with fluorouracil synergistic effect and SN-38 is an active metabolite of irinotecan.
No literature mainly on interactions with other drugs oxaliplatin
USE IN CASE OF PREGNANT AND LACTATION:
PREGNANCY: Level D
Unknown oxaliplatin is excreted in human milk. Be cautious for pregnant women and nursing mothers as other drug is excreted in breast milk.
Cause cancer, genetic mutations and infertility: Oxaliplatin is found mutagenic in mammalian chromosomes tested in the clinic. Although research on the likelihood of cancer have not been conducted, but oxaliplatin is considered as a carcinogen. Oxaliplatin toxicity on mice embryo or fetus. Oxaliplatin can cause miscarriage for pregnant women, therefore, should not be used during pregnancy or if you must use it to weigh the benefits and risk of the fetus, and to have the consent of the patient.
ADVERSE EFFECTS:
Nerve system:
Diseases of the peripheral sensory nerve can be increased, and dose-related diseases can be improved if the reduction or discontinuation of the drug. Neurological diseases are considered as special studies on neurotoxicity ladders have some changes compared to the usual standards of the National Cancer Institute, version 2. In the previous research, information neurological disease was collected announced that oxaliplatin is related to two types of neuropathy.
Acute syndrome of sensory nerves, quick recovery, belonging primarily peripheral neuropathy, the disease has a strong start, has manifested within 1 or 2 hours of use, and can be finished within 14 hours, may relapse while taking extra doses. Symptoms may occur early or if you experience severe cold temperatures or cold exposure and the patient may have signs paresthesia, disturbances and decreased sensation felt transient on hands, feet, around the mouth or throat . Jaw cramps, abnormal sensation in the tongue, dysarthria, severe eye pain and feeling chest also been observed. Acute syndrome of sensory nerves, fast recovery is found in approximately 56% patients on oxaliplatin coordination 5-FU / LV. In some cases, acute toxicity on nerve cyclical been observed in approximately 30% of patients. Ice (used in cases of talks) should be avoided during use of oxaliplatin, as cold temperatures can exacerbate acute symptoms of neuropathy.
Acute confusion syndrome feel pharynx-larynx region accounts for about 1 ~ 2% (a ¾) for patients with colorectal cancer have not been previously treated and untreated patients, this syndrome represents represented by subjective feelings as difficulty swallowing, shortness of breath without any objective evidence of laryngospasm, bronchospasm (no wheezing, no neck).
Sensory nerve disease persists, belonging primarily peripheral neuropathy (lasting> 14 days) is represented by the signs paresthesia, disturbances feeling, decreased sensation, but may include lack receptors for itself related to daily activities (such as discomfort in writing, sewing, yawning, travel). The symptoms of nerve occurs in approximately 48% of patients using oxaliplatin coordination 5-FU / LV. Persistent neuropathy can occur without any previous acute symptoms. The majority of patients (approximately 80%) usually occurs neuropathy progression by level 3 from level 1 or 2 symptoms earlier. Symptoms may improve if you stop treatment with oxaliplatin.
Hematology:
Reduce neutrophils, thrombocytopenia, anemia, and neutropenia can occur when using oxaliplatin. Incidence of all cases of dengue in the oxaliplatin group is generally higher than for the fluorouracil group. For cancer patients with metastatic colorectal using oxaliplatin, oxaliplatin / 5-FU / leucovorin or 5-FU / leucovorin, the incidence of grade ¾ impact on hematological include anemia (1% , 2%, 2%), neutropenia (0%, 19%, 1%), decreased neutrophils (0%, 44%, 5%) and thrombocytopenia (3%, 4%, 0% ), corresponding to the group. Decreased neutrophil fever were reported in 6% of patients with 5-fluorouracil use / leucovorin / oxaliplatin.
Digestive:
Nausea, vomiting, diarrhea, mucositis / gastritis, abdominal pain occur with patients receiving oxaliplatin. For cancer patients with colorectal metastases were treated with oxaliplatin, oxaliplatin / 5-FU / leucovorin or 5-FU / leucovorin, the proportion of patients with grade 3-4 toxicities included nausea (4% , 11%, 4%), vomiting (4%, 9%, 4%), diarrhea (4%, 11%, 3%) and gastritis (0%, 3%, 3%), corresponding to groups. Incidence of undesirable effects on the gastrointestinal system in patients with untreated and previously treated was seen as a cross-cycle. Recommended before using antiemetics, including blocking drugs 5-HT3 receptors. Diarrhea and mucositis may be more severe when the combination of oxaliplatin and 5-FU / LV, and should take measures to support appropriate treatment.
Skin:
There have been reports of limb syndrome, radiation-back, hair loss occurs when using oxaliplatin. Oxaliplatin did not increase the rate of hair loss compared to the monotherapy with 5-FU / LV. No full report on the case of hair loss.
Cardiovascular system:
Witness reported tachycardia is occurring for> 2% and <5% of patients with colorectal cancer metastases using oxaliplatin / 5-fluorouracil / leucovorin. Rarely phlebitis.
Pulmonary toxicity:
Oxaliplatin is considered to be related to pulmonary fibrosis (<1% of patients), which may be fatal. Overall incidence of cases of cough, dyspnea and hypoxemia is 43% (for all authoritarian) and 7% (3 and 4 toxicity) in the oxaliplatin group combines 5-FU / LV compared with 32% (all toxicity) va5% (toxicity 3 and 4) in the irinotecan group combines 5-FU / LV, the patients did not determine the duration of illness and untreated cancer-direct colon before. In the case of the respiratory symptoms of unknown etiology such as cough without phlegm, shortness of breath, wheezing, or signs of abnormal chest radiographs, oxaliplatin is not recommended until the review more screening test to exclude pulmonary interstitial lung disease or pulmonary fibrosis.
Allergic reactions:
The allergic reactions in nature and severity similar to other platinum containing compounds such as rash, urticaria, erythema, pruritus, and rarely bronchospasm and hypotension. These reactions are usually treated with epinephrine, corticosteroid, and antihistamine. For patients aged 65 years or older may be more prone to dehydration, diarrhea, hypokalemia and fatigue.
When used in conjunction with the oxaliplatin infusion 5-FU / LV; some side effects can occur in about> 2% and <5%. Namely anxiety, muscle pain, rash, increased sweating, conjunctivitis, weight loss, dry mouth, rectal hemorrhage, asthenia, ataxia, ascites, hemorrhoids, muscle weakness, restlessness , tachycardia, abnormal urination, dry skin, itching, coughing up blood, purpura, bleeding, black stool, drowsiness, lung diseases, inflammation of the rectum, involuntary muscle contractions, ileus , gingivitis, feeling a pain, heat, bloating and bedwetting. God:
Approximately 5-10% of patients in the group increases in serum creatinine clearance. The incidence of increased serum creatinine levels when using oxaliplatin ¾ and 5-FU / LV is 1% of the patients had been treated previously.
Liver:
Increased total bilirubin occurred> 5% of patients were treated oxaliplatin. Uncommon cases of transient increase in transaminases.
AFFECTING THE ABILITY TO DRIVE AND OPERATE MACHINERY:
Uninfluence
OVERDOSAGE AND MANAGEMENT:
No specific antidote in case of overdose. In general, supportive treatment should be monitored regularly and symptoms of overdose.
STORAGE CONDITIONS:
The drug still in the original packaging: Store at temperatures below 30 ° C.
After dilution solution: To ensure safety in terms of chemical and microbiological, diluted solution should be used immediately, in case of not using immediately, store in refrigerator (2-8oC) and use within the past 24 hours.
DOSAGE AND PRESENTATION:
Box 1 vial lyophilized powder for injection.
STANDARD: Manufacturer.
SHELF LIFE: 36 months from date of manufacture.
MADE BY:
CHONG KUN DANG PHARMACEUTICAL CORP.
===============================================
Distributor: LYNH FARMA CO., LTD.
49A30 Phan Dang Luu Street, Ward 7, Phu Nhuan District, HCMC.
Tel: +84 8 3510 87 57 Fax: +84 8 3510 87 56
Distributor: LYNH FARMA CO., LTD.
49A30 Phan Dang Luu Street, Ward 7, Phu Nhuan District, HCMC.
Tel: +84 8 3510 87 57 Fax: +84 8 3510 87 56
CKD BELLOXA INJECTION, 50MG
Each vial contains:
- Oxaliplatin ...........................50 mg
- Excipients: Lactose ..............450 mg
Each vial contains:
- Oxaliplatin ...........................50 mg
- Excipients: Lactose ..............450 mg
Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.




.jpg )