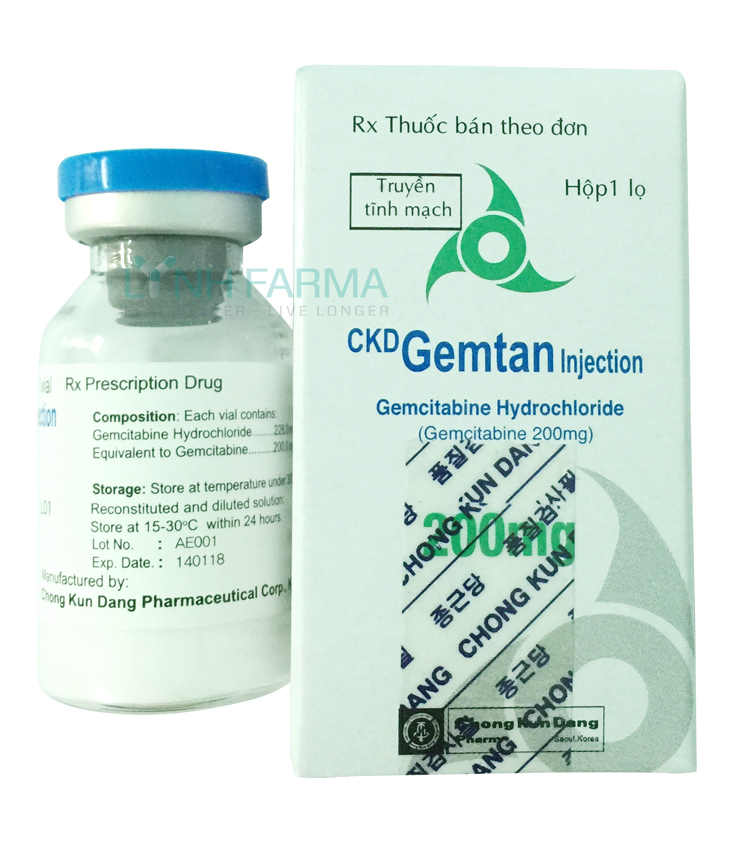
CKD GEMTAN Injection 200 mg
Dosage forms:
Lyophilized powder for injection. Intravenous drug use only.
Standard treatment:
- Lung cancer non-small cell.
- Pancreatic cancer.
- Bladder cancer.
- Breast cancer.
- Ovarian Cancer.
Price :
YOU NEED TO HELP ?
HOTLINE: 0917 55 70 88
- Company address: 49A30 Phan Dang Luu,Ward 7, Phu Nhuan District, Ho Chi Minh City, Vietnam.
- Email: info@lynhfarma.com
HOTLINE: 0917 55 70 88
- Company address: 49A30 Phan Dang Luu,Ward 7, Phu Nhuan District, Ho Chi Minh City, Vietnam.
- Email: info@lynhfarma.com
- Product Information
- Ingredient
CKD GEMTAN INJECTION 200mg.
Each vial contains:
- Gemcitabine hydrochloride.228,0 mg (Equivalent to 200.0 mg gemcitabine)
- Excipients: D-Mannitol 200.0 mg, 12.5 mg sodium acetat.3H2O, sufficient sodium hydroxide, hydrochloric acid enough.
DESCRIPTION DOSAGE: Lyophilized powder for injection white.
POINT:
Lung cancer, non-small cell
- Treatment with cisplatin combination leading to lung cancer, non-small cell has not spread or metastasize.
- Independent palliative care for cancer non-small cell lung metastasis or no metastasis.
Pancreatic cancer
- Treatment of pancreatic cancer is not leading or metastasis.
Bladder cancer
Breast cancer
- Use in combination with paclitaxel is indicated for the treatment of patients with breast cancer that has spread or not after treatment with chemotherapy containing anthracyclin anthracyclin if not contraindicated.
Ovarian Cancer
- Use in combination with carboplatin is indicated for the treatment of patients with ovarian cancer that has spread and recurrence of at least 6 months after completion of chemotherapy based on platinum.
DOSAGE AND USE:
Intravenous drug use only.
Standard treatment
1) Lung cancer non-small cell
Use single
(Adults): Gemcitabine given intravenously at a dose of 1,000 mg / m2 for 30 minutes, once a week. After 3 weeks of consecutive use, should stop taking the drug for a week. Repeat as above 4-week cycle. Adjust dose or dose level maintained depending on the level undesirable effects on patient appearance.
Using combinations
(Adults): 2 dose regimen combined with ciplastin. A dosage regime of cyclical 3 weeks and a 4-week cycle use.
With a 3-week dose regimen, gemcitabine 1,250 mg dose used in / m2 in 30 minutes on 1 and 8. Adjust dose or dose level maintained depending on the level undesirable effects on patient appearance.
With a 4-week dose regimen, gemcitabine dose of 1,000 mg / m2 for 30 minutes at day 1, 8 and 15. Adjusting the dose or dose rate depending keep level undesirable effects on patient appearance.
Cisplatin given intravenously at a dose of 75 ~ 100 mg / m2 every 3 or 4 weeks.
2) Pancreatic Cancer (Adults)
Gemcitabine given intravenously at a dose of 1,000 mg / m2 in 30 min / week for 7 weeks, followed by 1 week of treatment stops. The next cycle should take line 1 / week for 3 weeks in a row and stay next week. Adjust dose or dose level maintained depending on the level undesirable effects on patient appearance.
3) Bladder Cancer
Using combinations
(Adults) Combined with cisplatin, gemcitabine given intravenously at a dose of 1,000 mg / m2 for 30 minutes at days 1, 8 and 15 minutes on days 1, 8 and 15 of each cycle of 4 weeks, followed by 1 week Vacation treatment. Cisplatin at a dose of 70 mg/m2 on day 1 after using gemcitabine or day 2 of using cycles 4 weeks. 4 week treatment cycle can be repeated. Before each chemotherapy, a dose reduction or stop treatment based on the level of unwanted effects on patient appearance. According to the results of clinical trials, combination therapy with 100 mg / m2 of cisplatin significantly improved compared to marrow failure treated independently.
4) Breast cancer
Combination therapy
(Adults): intravenous dose gemcitabine 1,250 mg / m2 in 30 minutes on Day 1 and 8 of each 21 day cycle. Paclitaxel at a dose of 175 mg using / m2 on day 1 of each cycle intravenous gemcitabine 3 hours before using. Adjust dose or dose level maintained depending on the level undesirable effects on patient appearance. Patients should be monitored prior to each dose using the total number of each type of blood cells and blood cells. Patients should have the number of granulocytes ≥1.500 x 106 / L and platelet ≥100.000 x 106 / L.
5) Ovarian cancer.
Combination therapy
(Adults): gemcitabine given intravenously at a dose of 1,000 mg / m2 for 30 minutes a day 1 and 8 of each 21 day cycle. Carboplatin given intravenously at a dose of 40 ml / min and 1 day after taking gemcitabine. Patients need to be monitored prior to each dose of total blood cells and the number of each type of blood cell. Patients must have a number of granulocyte ≥1.500 x 106 / L and platelet ≥100.000 x 106 / L.
Adjusting the dose to dose reduction or end of treatment
Patients using gemcitabine should be controlled before the total number of blood cells and each cell, platelet. If hematological toxicity detection, need to adjust or stop using the drug as directed in Table 1.
Table 1. Guidance dose reduction.
Should conduct periodic tests of liver and kidney function including transaminases and serum creatinine before and after starting treatment. Doses should be reduced in each cycle or cycle on the extent of unwanted effects on patient appearance.
Elderly
Patients over age 65 can still tolerated gemcitabine. Although clearance and half-life of the drug is affected by age, there is no evidence that the need for dose adjustment for patients over 65 years. Generally, in studies using gemcitabine alone, the pace appears undesirable effect is almost equal in men and women, but women, especially elderly women do not have to conduct a cycle the next period and appeared phenomenon reduced neutrophil and platelet levels 3 and 4 more.
How to use:
- Only use 0.9% sodium chloride solution without preservatives for mixing gemcitabine sterile injectables. Although not perceived the incompatibility of the drug, gemcitabine should not be mixed with other medications containing. Due to the solubility of gemcitabine, gemcitabine phase with a maximum concentration of 40 mg / mL. Avoid drugs mixed in a concentration greater than 40 mg / mL for the drug can not be dissolved completely.
- In order to infusion, add at least 5 mL of 0.9% sodium chloride solution into the vial 200 mg and at least 25 mL of 0.9% sodium chloride solution in 1 g vials. Shake to dissolve.
Drug solution after dilution is clear solution, colorless. Check visually solution before use, using only the clear solution, free of debris or change the color of the drug.
-Can Caution when preparing the drug solution, should use gloves, if the drug solution in contact with skin or soft tissue, should wash the skin with soap and water or rinse with large amounts of soft tissue water.
CONTRAINDICATIONS:
1) Patients with hypersensitivity to gemcitabine.
2) Use in combination with cisplatin should be avoided in patients with impaired renal function seriously.
3) Do not use in patients with impaired liver function moderate to severe renal (glomerular filtration rate below 30 ml / min).
4) Patients with severe myelosuppression (can be fatal to aggravate the situation myelosuppression).
5) Patients with serious infections (May cause increased mortality due to infection).
6) Women who are pregnant or likely to become pregnant (In preclinical studies, the possibility of defects or fetal death have been reported).
THE WARNINGS AND PRECAUTIONS:
1) Extend the transmission time and increases frequency of use increases the toxicity of the drug.
2) Gemcitabine can cause bone marrow suppression and anemia, decreased neutrophil and can cause organ hemorrhage. Myelosuppression may occur transient; however, it should be strictly controlled during treatment. Patients with severe myelosuppression should not use gemcitabine.
3) When patients show signs of the early stages of hemolysis include rapid reduction of hemoglobin with bleeding, blood bilirubin, serum creatinine, blood urea nitrogen and increase LDH need to stop treatment with gemcitabine.
4) Pulmonary toxicity has been reported with use of gemcitabine. In the case of severe lung toxicity, treatment with gemcitabine should be stopped immediately and supportive treatment conducted. Patients should stop treatment when interstitial pneumonia or pulmonary fibrosis shown in clinical and X-ray images.
5) Hemolytic uremic syndrome and / or renal failure have been reported following use of a dose of gemcitabine or more. Renal failure leading to death or require dialysis have been reported but are rare. Most of the cases of renal failure leading to death were due to hemolytic uremic syndrome.
6) Severe liver toxicity including liver dysfunction and death have been reported but are rare when used alone or in combination with other drugs associated with liver toxicity.
General Precautions
1) Gemcitabine is used in these cases need to be treated by medical professionals with enough experience in cancer chemotherapy. Gemcitabine when used should be the consent of the patients after explaining the benefits and risks for the patient and family.
2) Patients treated gemcitabine should be controlled by a medical professional experienced in the use of cancer chemotherapy. Most of the undesirable effects that can restore and unnecessary interruption of therapy can be reduced even if the dose of the drug. Women, especially older women have greater ability not use an next cycle.
3) The drug is only used in intravenous lines. The drug does not cause necrosis response and ease of use. Reactions around the injection site necrosis occurs rarely and no reports of cases of scleroderma.
4) Patients using gemcitabine should be controlled and evaluated the basic routine tests.
5) Patients with spinal cord function impairment need to be cautious when using gemcitabine. Patients use a combination or successive chemotherapy should consider the risk increased dysfunction of the spinal cord like other chemotherapy drugs.
6) Patients using gemcitabine should be controlled before each dose of the total white blood cells, platelets and granulocytes. In the case of bone marrow toxicity caused by gemcitabine (see dosage and usage), it is necessary to adjust the dose or stop treatment. Even after stopping the drug, the number of blood cells could decrease continuously.
7) Patients with liver function, renal impairment should be cautious when using gemcitabine. There are no studies on the use of this drug in patients with impaired liver function and kidney. Heavy to moderate renal impairment (glomerular filtration rate of 30 to 80 ml / min) did not significantly affect the efficiency of the pharmacokinetics of gemcitabine. Liver and kidney function of patients should be monitored regularly by clinical tests.
8) Because the unwanted effects such as myelosuppression and interstitial pneumonia may occur and sometimes with severity, the clinical manifestations need to be controlled each use. At the same time, clinical tests (blood tests, liver and kidney function) and X - ray should be done regularly, and adjust the dose or stopping the drug may consider necessary.
9) Interstitial pneumonitis, pulmonary toxicity: The clinical signs should be strictly controlled (ventilation, coughing, fever, ...) and X - ray should be done regularly. If necessary, chest CT, arterial oxygen measurement and the ability to diffuse through the membrane lung needs to be done. Adjust dose or discontinue use may be considered if necessary.
INTERACTIONS WITH OTHER MEDICINES:
No data
USE IN CASE OF PREGNANT AND LACTATION:
Contraindicated for women who are pregnant or likely to become pregnant (In preclinical studies, the possibility of defects or fetal death have been reported).
DRUGS USED FOR CHILDREN
The efficacy of gemcitabine in children has not been proven. Gemcitabine was evaluated in phase 1 trials of childhood leukemia is persistent and determined the maximum tolerated dose of 10 mg / m2 / min for 360 minutes 3 times / week, then 1 week break. Gemcitabine was also evaluated in Phase 2 trials in patients with acute lymphoblastic leukemia (22 patients) and acute myeloid leukemia (10 patients) using 10 mg/m2/min 3 times/week and then a 1 week break. The toxicity observed included bone marrow suppression, decreased neutrophils, fever, increased blood transaminases, nausea and rash.
ADVERSE EFFECTS:
1) Blood and lymphatic system
It can cause dose-dependent myelosuppression may therefore appear anemia, leukopenia or thrombocytopenia, but less than 1% of patients stop treatment. 19% of patients required red blood transmission and 1% of patients had infections. 16% of patients with normal or mild bleeding. Thrombocytopenia is often reported, but only 1% of patients required platelet transfusions
2) Liver
Gemcitabine related to increased blood transaminase 1 of 2 in 70% of patients. The increase is not serious, the patient does not need to stop treatment. However, if patients with impaired hepatic function, caution should be exercised when using the drug. In phase 4 studies, impaired liver function and jaundice include increased ASAT, ALAT, ALP and bilirubin have been reported. Energy hepatotoxicity including hepatic dysfunction and mortality rarely been reported in patients who used alone or in combination with other drugs associated with liver toxicity.
3) Digestive
69% of patients reported using drugs gagging condition. 20% have symptoms without other treatment measures. However the need to reduce the dose cases are rare and symptoms easily removed by conventional anti-nausea drugs. Diarrhea, gastritis and ulcers in the mouth, constipation are reported regularly.
4) Respiratory system
Mild breathing difficulties in a short time or a few hours can occur, however, rarely need to reduce the dose, and most of the cases, the symptoms of loss without other drug therapy. patients with hypersensitivity to gemcitabine should not use this drug.
Pulmonary edema and respiratory distress syndrome in adults progression rarely been reported, and the cause has not yet been clarified. If these symptoms appear to stop taking the medication and treatment support. Sometimes pneumonia are reported with pulmonary infiltrates. In this case, the drug should be discontinued immediately and the symptoms may decrease when using steroids. Reduce blood oxygen, increase blood CO2, PIE syndrome, wheezing and short time may occur.
There have been reports of pulmonary edema and ARDS (respiratory distress syndrome adults) of unknown cause. If symptoms increase, should consider discontinuation of treatment should be considered gemcitabine.
5) Kidney
50% of patients reported using the drug situation proteinuria and hematuria severe, but most cases do not have a significant impact in the clinical studies. Most symptoms are rarely associated with blood creatinine or blood urea nitrate. However, renal failure of unknown etiology have been reported.
The clinical findings related to urea hemolytic syndrome were reported in 6 of 2429 patients (0.25%) using gemcitabine in clinical trials. Use gemcitabine should be stopped immediately if the patient has anemia with hemolytic IC expression, increased bilirubin or LDH, increase reticulocyte, severe thrombocytopenia, and / or kidney failure. Renal failure may not be reversible even after discontinuation of treatment and may require dialysis. Reducing the whole protein, electrolyte disorders (over 10%), decreased albumin, increased blood urea and creatinine, teens may appear.
6) Allergy / Hypersensitivity
Using drugs can be rash and itching. Most cases are not too severe erythema and not to reduce the dose, and can be treated on site. Sometimes it may appear peeling, cysts, sores. Anaphylactic reactions were reported rarely. In the case of anaphylactic reactions such as respiratory syndrome, patients should stop taking the drug immediately and take measures appropriate care. Bronchospasm were reported with a frequency of less than 1% (1 case). Symptoms are mild and transient, however, need to be treated. Do not use gemcitabine for patients with hypersensitivity to the drug.
7) Circulatory system
In the clinical trial, 2% of patients withdrew because of cardiovascular problems such as heart attack, stroke, hypertension and arrhythmias. Many of these patients had a history of cardiovascular disease before taking the drug. Myocardial infarction, congestive heart failure, arrhythmias are the unwanted effects, but the link between this disease and the drug is unknown. Some cases of hypotension have been reported. Heart palpitations, angina, palpitations, external ventricular, atrial and ventricular beats ECG disorders can appear.
8) Other unwanted effects
- Body: Most patients with mild symptoms of fatigue (19%). However, this situation did not last long and do not need to reduce the dose. Fever, headache, chills, muscle aches, fatigue and loss of appetite are common symptoms. Cough, rhinitis, malaise, sweating and insomnia are also common symptoms. Fever and fatigue may occur independently of each other. Fever may not be accompanied by infection. Acetaminophen can relieve symptoms.
- Consistent, especially peripheral edema were reported frequently and rarely agrees on the report. Because compliance is not heavy so no need to reduce the dose; however compliance can be painful. Compliance may soon lose when patients stop smoking. Edema mechanism is unknown. The relationship with heart failure, liver failure or renal insufficiency has not been confirmed.
- The cases of infection were reported for 16% of patients due to reduced immune function. Septic shock rarely been reported (<1%).
- Hair loss often mild and drowsiness were reported.
- The spill at the site of injection were reported in 4% of patients who use the drug. No reports on the phenomenon of hardening at the injection site. Cell swelling and no severe reactions at the injection site that does not happen spills were reported. These serious skin reactions including exfoliative blistering and rarely reported.
- Vascular disorders: clinical manifestations of peripheral vascular inflammation and necrosis are rarely reported.
- Toxicity on nerve: Approximately 10% of patients with mild allergic commitments under 1% suffering from severe allergic but committed relationship with gemcitabine have not been clarified.
- The table below shows the adverse effects occurring in ≥10% (at all levels) for ovarian cancer patients. Here are the unwanted effects occur in 1-10% (at all levels) patients. In parentheses is the effect at 3 and 4 (gemcitabine in combination with carboplatin versus carboplatin): increased ASAT or ALAT (0 versus 1.2%), dyspnea (3.4% versus 2.9%) , decreased neutrophils, fever (1.1% vs 0), bleeding (2.3% vs 1.1%), hypersensitivity reactions (2.3% versus 2.9%) , hyperactivity (1.1% versus 0.6%) and rash (0.6% vs 0).
AFFECTING THE ABILITY TO DRIVE AND OPERATE MACHINERY:
Because the drug can cause drowsiness status caution should be exercised when driving and operating machinery.
OVERDOSAGE AND MANAGEMENT:
There is no specific treatment for overdose cases gemcitabine. When high-dose intravenous infusion of 5.7 g / m2 in 30 minutes 2 weeks / times, they show no significant toxicity in clinical practice. In the case of suspected overdose, patients should be monitored and treated CBC support if necessary
CONDITIONS OF STORAGE: Store at temperatures below 30 ° C.
After dilution solution: Store at 15-30oC temperature not exceeding 24 hours.
DOSAGE AND PRESENTATION: Box of 1 vial.
STANDARD: USP 35
SHELF LIFE: 36 months from date of manufacture.
MADE BY:
CHONG KUN DANG PHARMACEUTICAL CORP
- This drug is for a doctor's prescription.
- Carefully read the manual before use. If you need further information, please consult your doctor or pharmacist.
- Inform your physician about any adverse effects encountered during the treatment.
- To medicines out of reach of children.
- No medication overdue printed on the packaging.
Each vial contains:
- Gemcitabine hydrochloride.228,0 mg (Equivalent to 200.0 mg gemcitabine)
- Excipients: D-Mannitol 200.0 mg, 12.5 mg sodium acetat.3H2O, sufficient sodium hydroxide, hydrochloric acid enough.
DESCRIPTION DOSAGE: Lyophilized powder for injection white.
POINT:
Lung cancer, non-small cell
- Treatment with cisplatin combination leading to lung cancer, non-small cell has not spread or metastasize.
- Independent palliative care for cancer non-small cell lung metastasis or no metastasis.
Pancreatic cancer
- Treatment of pancreatic cancer is not leading or metastasis.
Bladder cancer
Breast cancer
- Use in combination with paclitaxel is indicated for the treatment of patients with breast cancer that has spread or not after treatment with chemotherapy containing anthracyclin anthracyclin if not contraindicated.
Ovarian Cancer
- Use in combination with carboplatin is indicated for the treatment of patients with ovarian cancer that has spread and recurrence of at least 6 months after completion of chemotherapy based on platinum.
DOSAGE AND USE:
Intravenous drug use only.
Standard treatment
1) Lung cancer non-small cell
Use single
(Adults): Gemcitabine given intravenously at a dose of 1,000 mg / m2 for 30 minutes, once a week. After 3 weeks of consecutive use, should stop taking the drug for a week. Repeat as above 4-week cycle. Adjust dose or dose level maintained depending on the level undesirable effects on patient appearance.
Using combinations
(Adults): 2 dose regimen combined with ciplastin. A dosage regime of cyclical 3 weeks and a 4-week cycle use.
With a 3-week dose regimen, gemcitabine 1,250 mg dose used in / m2 in 30 minutes on 1 and 8. Adjust dose or dose level maintained depending on the level undesirable effects on patient appearance.
With a 4-week dose regimen, gemcitabine dose of 1,000 mg / m2 for 30 minutes at day 1, 8 and 15. Adjusting the dose or dose rate depending keep level undesirable effects on patient appearance.
Cisplatin given intravenously at a dose of 75 ~ 100 mg / m2 every 3 or 4 weeks.
2) Pancreatic Cancer (Adults)
Gemcitabine given intravenously at a dose of 1,000 mg / m2 in 30 min / week for 7 weeks, followed by 1 week of treatment stops. The next cycle should take line 1 / week for 3 weeks in a row and stay next week. Adjust dose or dose level maintained depending on the level undesirable effects on patient appearance.
3) Bladder Cancer
Using combinations
(Adults) Combined with cisplatin, gemcitabine given intravenously at a dose of 1,000 mg / m2 for 30 minutes at days 1, 8 and 15 minutes on days 1, 8 and 15 of each cycle of 4 weeks, followed by 1 week Vacation treatment. Cisplatin at a dose of 70 mg/m2 on day 1 after using gemcitabine or day 2 of using cycles 4 weeks. 4 week treatment cycle can be repeated. Before each chemotherapy, a dose reduction or stop treatment based on the level of unwanted effects on patient appearance. According to the results of clinical trials, combination therapy with 100 mg / m2 of cisplatin significantly improved compared to marrow failure treated independently.
4) Breast cancer
Combination therapy
(Adults): intravenous dose gemcitabine 1,250 mg / m2 in 30 minutes on Day 1 and 8 of each 21 day cycle. Paclitaxel at a dose of 175 mg using / m2 on day 1 of each cycle intravenous gemcitabine 3 hours before using. Adjust dose or dose level maintained depending on the level undesirable effects on patient appearance. Patients should be monitored prior to each dose using the total number of each type of blood cells and blood cells. Patients should have the number of granulocytes ≥1.500 x 106 / L and platelet ≥100.000 x 106 / L.
5) Ovarian cancer.
Combination therapy
(Adults): gemcitabine given intravenously at a dose of 1,000 mg / m2 for 30 minutes a day 1 and 8 of each 21 day cycle. Carboplatin given intravenously at a dose of 40 ml / min and 1 day after taking gemcitabine. Patients need to be monitored prior to each dose of total blood cells and the number of each type of blood cell. Patients must have a number of granulocyte ≥1.500 x 106 / L and platelet ≥100.000 x 106 / L.
Adjusting the dose to dose reduction or end of treatment
Patients using gemcitabine should be controlled before the total number of blood cells and each cell, platelet. If hematological toxicity detection, need to adjust or stop using the drug as directed in Table 1.
Table 1. Guidance dose reduction.
|
Number of granulocyte (x106/L) |
|
Platelet count (x106/L) |
% Normal dose |
|
> 1.000 500 ~ 1.000 < 500
|
And Or Or |
> 100.000 50.000 ~ 100.000 < 50.000 |
100% 75% Pause |
Elderly
Patients over age 65 can still tolerated gemcitabine. Although clearance and half-life of the drug is affected by age, there is no evidence that the need for dose adjustment for patients over 65 years. Generally, in studies using gemcitabine alone, the pace appears undesirable effect is almost equal in men and women, but women, especially elderly women do not have to conduct a cycle the next period and appeared phenomenon reduced neutrophil and platelet levels 3 and 4 more.
How to use:
- Only use 0.9% sodium chloride solution without preservatives for mixing gemcitabine sterile injectables. Although not perceived the incompatibility of the drug, gemcitabine should not be mixed with other medications containing. Due to the solubility of gemcitabine, gemcitabine phase with a maximum concentration of 40 mg / mL. Avoid drugs mixed in a concentration greater than 40 mg / mL for the drug can not be dissolved completely.
- In order to infusion, add at least 5 mL of 0.9% sodium chloride solution into the vial 200 mg and at least 25 mL of 0.9% sodium chloride solution in 1 g vials. Shake to dissolve.
Drug solution after dilution is clear solution, colorless. Check visually solution before use, using only the clear solution, free of debris or change the color of the drug.
-Can Caution when preparing the drug solution, should use gloves, if the drug solution in contact with skin or soft tissue, should wash the skin with soap and water or rinse with large amounts of soft tissue water.
CONTRAINDICATIONS:
1) Patients with hypersensitivity to gemcitabine.
2) Use in combination with cisplatin should be avoided in patients with impaired renal function seriously.
3) Do not use in patients with impaired liver function moderate to severe renal (glomerular filtration rate below 30 ml / min).
4) Patients with severe myelosuppression (can be fatal to aggravate the situation myelosuppression).
5) Patients with serious infections (May cause increased mortality due to infection).
6) Women who are pregnant or likely to become pregnant (In preclinical studies, the possibility of defects or fetal death have been reported).
THE WARNINGS AND PRECAUTIONS:
1) Extend the transmission time and increases frequency of use increases the toxicity of the drug.
2) Gemcitabine can cause bone marrow suppression and anemia, decreased neutrophil and can cause organ hemorrhage. Myelosuppression may occur transient; however, it should be strictly controlled during treatment. Patients with severe myelosuppression should not use gemcitabine.
3) When patients show signs of the early stages of hemolysis include rapid reduction of hemoglobin with bleeding, blood bilirubin, serum creatinine, blood urea nitrogen and increase LDH need to stop treatment with gemcitabine.
4) Pulmonary toxicity has been reported with use of gemcitabine. In the case of severe lung toxicity, treatment with gemcitabine should be stopped immediately and supportive treatment conducted. Patients should stop treatment when interstitial pneumonia or pulmonary fibrosis shown in clinical and X-ray images.
5) Hemolytic uremic syndrome and / or renal failure have been reported following use of a dose of gemcitabine or more. Renal failure leading to death or require dialysis have been reported but are rare. Most of the cases of renal failure leading to death were due to hemolytic uremic syndrome.
6) Severe liver toxicity including liver dysfunction and death have been reported but are rare when used alone or in combination with other drugs associated with liver toxicity.
General Precautions
1) Gemcitabine is used in these cases need to be treated by medical professionals with enough experience in cancer chemotherapy. Gemcitabine when used should be the consent of the patients after explaining the benefits and risks for the patient and family.
2) Patients treated gemcitabine should be controlled by a medical professional experienced in the use of cancer chemotherapy. Most of the undesirable effects that can restore and unnecessary interruption of therapy can be reduced even if the dose of the drug. Women, especially older women have greater ability not use an next cycle.
3) The drug is only used in intravenous lines. The drug does not cause necrosis response and ease of use. Reactions around the injection site necrosis occurs rarely and no reports of cases of scleroderma.
4) Patients using gemcitabine should be controlled and evaluated the basic routine tests.
5) Patients with spinal cord function impairment need to be cautious when using gemcitabine. Patients use a combination or successive chemotherapy should consider the risk increased dysfunction of the spinal cord like other chemotherapy drugs.
6) Patients using gemcitabine should be controlled before each dose of the total white blood cells, platelets and granulocytes. In the case of bone marrow toxicity caused by gemcitabine (see dosage and usage), it is necessary to adjust the dose or stop treatment. Even after stopping the drug, the number of blood cells could decrease continuously.
7) Patients with liver function, renal impairment should be cautious when using gemcitabine. There are no studies on the use of this drug in patients with impaired liver function and kidney. Heavy to moderate renal impairment (glomerular filtration rate of 30 to 80 ml / min) did not significantly affect the efficiency of the pharmacokinetics of gemcitabine. Liver and kidney function of patients should be monitored regularly by clinical tests.
8) Because the unwanted effects such as myelosuppression and interstitial pneumonia may occur and sometimes with severity, the clinical manifestations need to be controlled each use. At the same time, clinical tests (blood tests, liver and kidney function) and X - ray should be done regularly, and adjust the dose or stopping the drug may consider necessary.
9) Interstitial pneumonitis, pulmonary toxicity: The clinical signs should be strictly controlled (ventilation, coughing, fever, ...) and X - ray should be done regularly. If necessary, chest CT, arterial oxygen measurement and the ability to diffuse through the membrane lung needs to be done. Adjust dose or discontinue use may be considered if necessary.
INTERACTIONS WITH OTHER MEDICINES:
No data
USE IN CASE OF PREGNANT AND LACTATION:
Contraindicated for women who are pregnant or likely to become pregnant (In preclinical studies, the possibility of defects or fetal death have been reported).
DRUGS USED FOR CHILDREN
The efficacy of gemcitabine in children has not been proven. Gemcitabine was evaluated in phase 1 trials of childhood leukemia is persistent and determined the maximum tolerated dose of 10 mg / m2 / min for 360 minutes 3 times / week, then 1 week break. Gemcitabine was also evaluated in Phase 2 trials in patients with acute lymphoblastic leukemia (22 patients) and acute myeloid leukemia (10 patients) using 10 mg/m2/min 3 times/week and then a 1 week break. The toxicity observed included bone marrow suppression, decreased neutrophils, fever, increased blood transaminases, nausea and rash.
ADVERSE EFFECTS:
1) Blood and lymphatic system
It can cause dose-dependent myelosuppression may therefore appear anemia, leukopenia or thrombocytopenia, but less than 1% of patients stop treatment. 19% of patients required red blood transmission and 1% of patients had infections. 16% of patients with normal or mild bleeding. Thrombocytopenia is often reported, but only 1% of patients required platelet transfusions
2) Liver
Gemcitabine related to increased blood transaminase 1 of 2 in 70% of patients. The increase is not serious, the patient does not need to stop treatment. However, if patients with impaired hepatic function, caution should be exercised when using the drug. In phase 4 studies, impaired liver function and jaundice include increased ASAT, ALAT, ALP and bilirubin have been reported. Energy hepatotoxicity including hepatic dysfunction and mortality rarely been reported in patients who used alone or in combination with other drugs associated with liver toxicity.
3) Digestive
69% of patients reported using drugs gagging condition. 20% have symptoms without other treatment measures. However the need to reduce the dose cases are rare and symptoms easily removed by conventional anti-nausea drugs. Diarrhea, gastritis and ulcers in the mouth, constipation are reported regularly.
4) Respiratory system
Mild breathing difficulties in a short time or a few hours can occur, however, rarely need to reduce the dose, and most of the cases, the symptoms of loss without other drug therapy. patients with hypersensitivity to gemcitabine should not use this drug.
Pulmonary edema and respiratory distress syndrome in adults progression rarely been reported, and the cause has not yet been clarified. If these symptoms appear to stop taking the medication and treatment support. Sometimes pneumonia are reported with pulmonary infiltrates. In this case, the drug should be discontinued immediately and the symptoms may decrease when using steroids. Reduce blood oxygen, increase blood CO2, PIE syndrome, wheezing and short time may occur.
There have been reports of pulmonary edema and ARDS (respiratory distress syndrome adults) of unknown cause. If symptoms increase, should consider discontinuation of treatment should be considered gemcitabine.
5) Kidney
50% of patients reported using the drug situation proteinuria and hematuria severe, but most cases do not have a significant impact in the clinical studies. Most symptoms are rarely associated with blood creatinine or blood urea nitrate. However, renal failure of unknown etiology have been reported.
The clinical findings related to urea hemolytic syndrome were reported in 6 of 2429 patients (0.25%) using gemcitabine in clinical trials. Use gemcitabine should be stopped immediately if the patient has anemia with hemolytic IC expression, increased bilirubin or LDH, increase reticulocyte, severe thrombocytopenia, and / or kidney failure. Renal failure may not be reversible even after discontinuation of treatment and may require dialysis. Reducing the whole protein, electrolyte disorders (over 10%), decreased albumin, increased blood urea and creatinine, teens may appear.
6) Allergy / Hypersensitivity
Using drugs can be rash and itching. Most cases are not too severe erythema and not to reduce the dose, and can be treated on site. Sometimes it may appear peeling, cysts, sores. Anaphylactic reactions were reported rarely. In the case of anaphylactic reactions such as respiratory syndrome, patients should stop taking the drug immediately and take measures appropriate care. Bronchospasm were reported with a frequency of less than 1% (1 case). Symptoms are mild and transient, however, need to be treated. Do not use gemcitabine for patients with hypersensitivity to the drug.
7) Circulatory system
In the clinical trial, 2% of patients withdrew because of cardiovascular problems such as heart attack, stroke, hypertension and arrhythmias. Many of these patients had a history of cardiovascular disease before taking the drug. Myocardial infarction, congestive heart failure, arrhythmias are the unwanted effects, but the link between this disease and the drug is unknown. Some cases of hypotension have been reported. Heart palpitations, angina, palpitations, external ventricular, atrial and ventricular beats ECG disorders can appear.
8) Other unwanted effects
- Body: Most patients with mild symptoms of fatigue (19%). However, this situation did not last long and do not need to reduce the dose. Fever, headache, chills, muscle aches, fatigue and loss of appetite are common symptoms. Cough, rhinitis, malaise, sweating and insomnia are also common symptoms. Fever and fatigue may occur independently of each other. Fever may not be accompanied by infection. Acetaminophen can relieve symptoms.
- Consistent, especially peripheral edema were reported frequently and rarely agrees on the report. Because compliance is not heavy so no need to reduce the dose; however compliance can be painful. Compliance may soon lose when patients stop smoking. Edema mechanism is unknown. The relationship with heart failure, liver failure or renal insufficiency has not been confirmed.
- The cases of infection were reported for 16% of patients due to reduced immune function. Septic shock rarely been reported (<1%).
- Hair loss often mild and drowsiness were reported.
- The spill at the site of injection were reported in 4% of patients who use the drug. No reports on the phenomenon of hardening at the injection site. Cell swelling and no severe reactions at the injection site that does not happen spills were reported. These serious skin reactions including exfoliative blistering and rarely reported.
- Vascular disorders: clinical manifestations of peripheral vascular inflammation and necrosis are rarely reported.
- Toxicity on nerve: Approximately 10% of patients with mild allergic commitments under 1% suffering from severe allergic but committed relationship with gemcitabine have not been clarified.
- The table below shows the adverse effects occurring in ≥10% (at all levels) for ovarian cancer patients. Here are the unwanted effects occur in 1-10% (at all levels) patients. In parentheses is the effect at 3 and 4 (gemcitabine in combination with carboplatin versus carboplatin): increased ASAT or ALAT (0 versus 1.2%), dyspnea (3.4% versus 2.9%) , decreased neutrophils, fever (1.1% vs 0), bleeding (2.3% vs 1.1%), hypersensitivity reactions (2.3% versus 2.9%) , hyperactivity (1.1% versus 0.6%) and rash (0.6% vs 0).
AFFECTING THE ABILITY TO DRIVE AND OPERATE MACHINERY:
Because the drug can cause drowsiness status caution should be exercised when driving and operating machinery.
OVERDOSAGE AND MANAGEMENT:
There is no specific treatment for overdose cases gemcitabine. When high-dose intravenous infusion of 5.7 g / m2 in 30 minutes 2 weeks / times, they show no significant toxicity in clinical practice. In the case of suspected overdose, patients should be monitored and treated CBC support if necessary
CONDITIONS OF STORAGE: Store at temperatures below 30 ° C.
After dilution solution: Store at 15-30oC temperature not exceeding 24 hours.
DOSAGE AND PRESENTATION: Box of 1 vial.
STANDARD: USP 35
SHELF LIFE: 36 months from date of manufacture.
MADE BY:
CHONG KUN DANG PHARMACEUTICAL CORP
- This drug is for a doctor's prescription.
- Carefully read the manual before use. If you need further information, please consult your doctor or pharmacist.
- Inform your physician about any adverse effects encountered during the treatment.
- To medicines out of reach of children.
- No medication overdue printed on the packaging.
===============================================
Distributor: LYNH FARMA CO., LTD.
49A30 Phan Dang Luu Street, Ward 7, Phu Nhuan District, HCMC.
Tel: +84 8 3510 87 57 Fax: +84 8 3510 87 56
Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.





.jpg )